Table of Contents
Before exploring how batteries are categorized, it’s fundamental to answer the foundational question: what is a battery? Simply put, a battery is an electrochemical device that stores chemical energy and converts it into electrical energy when connected to a circuit, powering everything from small gadgets to large vehicles. Batteries are classified based on various criteria including chemical composition, rechargeability, size, and application. Understanding these classifications is essential for selecting the right battery for specific applications, whether in consumer electronics, electric vehicles, or large-scale battery storage systems—all of which rely on the core energy-conversion function defined by “what is a battery”.
By Rechargeability
-
Primary Batteries
Non-rechargeable batteries designed for single use. They convert chemical energy to electrical energy through irreversible reactions. Examples include alkaline batteries, zinc-carbon batteries, and lithium primary batteries commonly used in remote controls, flashlights, and smoke detectors.
-
Secondary Batteries
Rechargeable batteries that can undergo multiple charge-discharge cycles. They are essential components in battery storage systems, electric vehicles, smartphones, and laptops. The reversible chemical reactions allow these batteries to be recharged hundreds or thousands of times.

Different battery types used in various applications
By Chemical Composition
| Battery Type | Key Characteristics | Common Applications |
|---|---|---|
| Lead-Acid | Low cost, mature technology, heavy weight | Automotive starting batteries, uninterruptible power supplies, small-scale battery storage |
| Nickel-Cadmium (NiCd) | Good cycle life, suffers from memory effect | Portable power tools, emergency lighting |
| Nickel-Metal Hydride (NiMH) | Higher energy density than NiCd, less memory effect | Hybrid vehicles, digital cameras, portable electronics |
| Lithium-Ion (Li-ion) | High energy density, lightweight, no memory effect | Smartphones, laptops, electric vehicles, battery storage systems |
| Lithium-Ion Polymer (LiPo) | Flexible form factor, high energy density | Tablets, drones, portable media players |
Among these, lithium-ion batteries have become the dominant technology in modern applications due to their superior energy density and cycle life. They are particularly crucial for battery storage systems that integrate renewable energy sources like solar and wind, addressing the intermittent nature of these power sources.
Other specialized battery types include flow batteries, which are gaining attention for large-scale stationary battery storage applications due to their long cycle life and scalability, and solid-state batteries, an emerging technology promising higher energy densities and improved safety compared to conventional lithium-ion batteries.
Key Insight
The choice of battery chemistry depends on specific application requirements including energy density, power density, cycle life, cost, and operating temperature range. For battery storage applications, cycle life and cost are often more critical factors than for consumer electronics where energy density may be prioritized.
The conversion between chemical energy and electrical energy in batteries relies on electrochemical reactions involving the transfer of electrons—key to understanding how do batteries work. This fundamental process enables the storage and release of electrical energy in a portable form, making battery storage systems possible for numerous applications.
The Electrochemical Cell
A basic battery consists of one or more electrochemical cells, each containing two electrodes (anode and cathode) immersed in an electrolyte. The key principle involves spontaneous redox (reduction-oxidation) reactions:
- 1 Oxidation occurs at the anode, where atoms lose electrons, becoming positively charged ions that move into the electrolyte.
- 2 Electrons flow from the anode through an external circuit to the cathode, creating an electric current that can power devices.
- 3 Reduction occurs at the cathode, where ions from the electrolyte gain electrons, completing the circuit.
Electrochemical cell operation principle
Discharge and Charge Cycles
Discharge Process
During discharge, the battery supplies electrical energy to an external load. The spontaneous chemical reactions proceed, converting stored chemical energy into electrical energy. Electrons flow from the anode to the cathode through the external circuit, powering devices or contributing to battery storage systems.
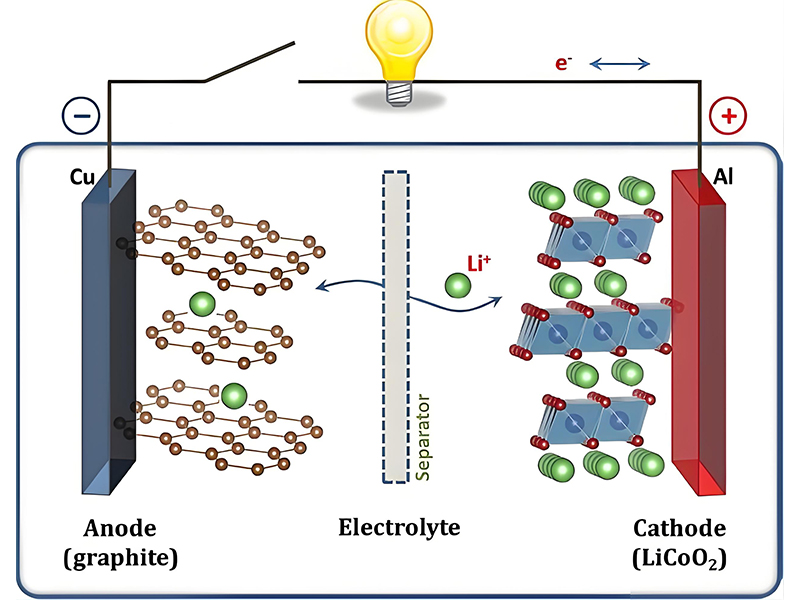
In a lithium-ion battery, during discharge, lithium ions move from the anode (typically graphite) to the cathode (often lithium metal oxide) through the electrolyte, while electrons flow through the external circuit to power devices.
Charge Process
For rechargeable batteries, an external electrical power source reverses the discharge process. Electrical energy is converted back into chemical energy, restoring the battery's capacity. This non-spontaneous reaction requires an external voltage greater than the battery's open-circuit voltage.
During charging of lithium-ion batteries, lithium ions are forced to move from the cathode back to the anode, where they are stored until the next discharge cycle. This reversible process enables the repeated use essential for effective battery storage solutions.
Energy Conversion Efficiency
No energy conversion process is 100% efficient, and batteries lose some energy as heat during both charge and discharge cycles. This efficiency is a critical factor in battery storage systems, as it directly impacts overall system performance and cost-effectiveness.
Comparison of typical round-trip efficiency for different battery storage technologies
Key Thermodynamic Principles
The voltage produced by a battery is determined by the Gibbs free energy change (ΔG) of the chemical reactions occurring within it. The relationship between the electrical potential (E) and the Gibbs free energy is given by:
Where:
- ΔG is the Gibbs free energy change (in joules)
- n is the number of moles of electrons transferred
- F is Faraday's constant (96,485 C/mol)
- E is the cell potential (in volts)
This fundamental relationship governs the maximum possible voltage a battery can produce and helps explain why different battery chemistries have characteristic voltage outputs. For battery storage applications, understanding these thermodynamic principles is essential for optimizing system design and performance.
Technical Note
The rate of electrochemical reactions in batteries is influenced by temperature, with higher temperatures generally increasing reaction rates but potentially reducing cycle life. This temperature sensitivity necessitates thermal management systems in many battery storage applications, particularly in electric vehicles and large-scale energy storage installations.
All batteries, regardless of type or application, share fundamental components that enable their electrochemical function. These components work together to facilitate the energy conversion process essential for battery storage systems. Understanding battery composition, which addresses what is inside a battery, is key to appreciating their performance characteristics and limitations.
Key Battery Components
The building blocks that enable energy storage and conversion
Anode (Negative Electrode)
The anode is the electrode where oxidation occurs during discharge, releasing electrons to the external circuit. In rechargeable batteries, it acts as the cathode during charging. Common anode materials include:
- Graphite in lithium-ion batteries
- Lead in lead-acid batteries
- Nickel-hydroxide in NiMH batteries
- Zinc in primary alkaline batteries
Cathode (Positive Electrode)
The cathode is where reduction occurs during discharge, accepting electrons from the external circuit. During charging, it functions as the anode. Typical cathode materials include:
- Lithium cobalt oxide (LiCoO₂) in consumer electronics
- Lithium iron phosphate (LiFePO₄) in battery storage systems
- Lead dioxide in lead-acid batteries
- Manganese dioxide in alkaline batteries
Electrolyte
The electrolyte is a medium that allows the flow of ions between the anode and cathode while preventing direct electron flow. It can be:
- Liquid (organic solvents in Li-ion batteries)
- Gel (in some sealed lead-acid batteries)
- Solid (in emerging solid-state battery technologies)
- Aqueous solutions (in lead-acid and alkaline batteries)
Additional Components
Separator
A physical barrier between the anode and cathode that prevents electrical short circuits while allowing ion passage. Separators are typically porous membranes made from polymers or ceramics, critical for battery safety and performance in all battery storage applications.
Case/Container
The outer enclosure that houses all battery components, providing mechanical protection and electrical insulation. Cases are designed to be durable while sometimes incorporating features for heat dissipation, important for battery storage systems operating under heavy loads.
Current Collectors
Conductive materials (usually metal foils) that collect electrons from the anode and deliver electrons to the cathode, facilitating current flow to and from the external circuit in all battery storage devices.
Battery Management System (BMS)
In modern rechargeable batteries, particularly lithium-ion systems used in battery storage, a BMS monitors and controls various parameters including voltage, current, and temperature. It prevents overcharging and over-discharging, balances cells in a battery pack, and ensures safe operation under all conditions. Advanced BMS can also provide state-of-charge estimation and communicate with external systems.
Safety Features
Many modern batteries incorporate safety mechanisms such as pressure relief valves, thermal fuses, and shutdown separators that activate under abnormal conditions. These features are particularly important for lithium-ion batteries used in battery storage systems, electric vehicles, and consumer electronics to prevent thermal runaway and potential safety hazards.
Material Selection Considerations
The choice of materials for each battery component depends on multiple factors that impact overall performance, particularly in battery storage applications:
| Consideration Factor | Description | Impact on Performance |
|---|---|---|
| Electrochemical Potential | The voltage difference between electrode materials | Determines battery voltage and energy density |
| Ion Conductivity | Ability of ions to move through electrolyte and electrodes | Affects charge/discharge rates and efficiency |
| Electrical Conductivity | Ability of electrodes to conduct electrons | Influences internal resistance and power output |
| Chemical Stability | Resistance to degradation during cycling | Determines cycle life and safety characteristics |
| Cost and Availability | Material cost and supply chain considerations | Impacts commercial viability, especially for large battery storage systems |
Advances in battery technology often involve developing new materials for these components to improve energy density, cycle life, safety, and reduce costs. Research into novel electrode materials, solid-state electrolytes, and advanced separators continues to push the boundaries of what's possible in battery storage technology, enabling new applications and improving existing ones.
Material Innovation Spotlight
Recent research into silicon anodes has shown promise for significantly increasing lithium-ion battery energy density, as silicon can store up to 10 times more lithium than graphite. However, challenges with volume expansion during cycling must be overcome before commercialization. Such innovations are critical for advancing battery storage capabilities in electric vehicles and renewable energy systems.
While individual battery cells provide specific electrical characteristics, most applications require multiple cells combined into battery packs to meet voltage, current, and capacity requirements, such as in a battery bank. This is particularly true for battery storage systems, electric vehicles, and other high-power applications that demand more energy than a single cell can provide.
Individual Battery Cells
A single battery cell is the basic building block, consisting of the anode, cathode, electrolyte, separator, and casing as discussed in the previous section. Each cell chemistry has characteristic nominal voltage and capacity:
Cell sizes vary widely, from small button cells used in watches to large prismatic cells designed for battery storage systems. Common form factors include cylindrical (like the 18650 and 21700 cells used in many applications), prismatic (flat, rectangular cases), and pouch cells (flexible, lightweight designs).
Different battery cell form factors
Battery Pack Configuration
Battery packs combine multiple cells through series and parallel connections to achieve desired voltage and capacity levels. This flexibility allows battery storage systems to be customized for specific applications, from small portable devices to large-scale energy storage installations.
Series Connection
- Increases total voltage (sum of individual cell voltages)
- Capacity remains the same as individual cells
- Example: 3 x 3.7V cells = 11.1V total
- Used to achieve higher voltage requirements in battery storage systems
Parallel Connection
- Voltage remains the same as individual cells
- Increases total capacity (sum of individual cell capacities)
- Example: 3 x 2Ah cells = 6Ah total at 3.7V
- Used to increase runtime and current output capability in battery storage applications
Series-Parallel Combinations
Most battery packs, especially those in battery storage systems and electric vehicles, use a combination of series and parallel connections to achieve both the required voltage and capacity. This configuration is often described with a "S-P" notation, where S represents the number of cells in series and P represents the number in parallel.
For example, a 3S2P configuration in a lithium-ion battery pack:
- 3 cells in series (3S) produce 11.1V (3 × 3.7V)
- 2 parallel groups (2P) double the capacity
- If each cell is 2Ah, total capacity becomes 4Ah
- Total energy: 11.1V × 4Ah = 44.4Wh
Complex battery storage systems can have hundreds or thousands of cells in intricate series-parallel configurations to achieve the high voltages (often hundreds of volts) and large capacities (many kilowatt-hours) required for applications like electric vehicles, backup power systems, and grid-scale energy storage.
Battery Pack Design Considerations
Cell Balancing
Due to slight manufacturing variations, cells in a pack may charge and discharge at different rates. Battery management systems (BMS) include cell balancing circuits to ensure all cells operate within similar voltage ranges, maximizing pack capacity and lifespan—critical for reliable battery storage performance.
Thermal Management
Uniform temperature distribution across cells is essential for performance and safety. Battery packs often include cooling systems (air, liquid, or phase-change materials) to maintain optimal operating temperatures, particularly important in high-power battery storage applications.
Mechanical Design
The physical arrangement must protect cells from vibration, shock, and mechanical stress while allowing for thermal management. Structural integrity is particularly important in automotive and portable battery storage applications.
Pack Electronics
Beyond the BMS, packs may include connectors, fuses, contactors, and communication interfaces that enable integration with external systems, monitoring, and control—essential features for modern battery storage systems.
Modern battery pack showing cell arrangement and thermal management features
The trend toward larger battery storage systems, particularly for renewable energy integration and electric mobility, has driven innovations in pack design. These include modular architectures that allow for scalability, improved thermal management systems for better performance, and advanced BMS with predictive analytics to optimize battery life and safety. As battery storage technology continues to evolve, pack design innovations will play a crucial role in maximizing system efficiency, reliability, and cost-effectiveness.
Industry Application
Electric vehicle battery packs represent some of the most sophisticated battery storage systems, often containing thousands of individual cells. For example, a typical electric vehicle battery pack might have a 400V+ system voltage and energy capacity ranging from 40kWh to over 100kWh, requiring complex series-parallel configurations and advanced management systems to ensure safe, efficient operation over many years of service.
Understanding battery parameters is essential for selecting the right battery for a specific application, including battery energy storage, and designing effective battery storage systems. These parameters define a battery's performance characteristics, limitations, and suitability for different uses, from small consumer electronics to large-scale energy storage installations.
Voltage
Voltage is the electrical potential difference between a battery's positive and negative terminals, measured in volts (V). It represents the force that drives electric current through a circuit.
- Nominal Voltage: Typical operating voltage (e.g., 3.7V for Li-ion)
- Open-Circuit Voltage: Voltage with no load applied
- Cut-off Voltage: Minimum voltage before damage occurs
- Peak Voltage: Maximum voltage during charging
Capacity
Capacity measures the total amount of electrical charge a battery can store, typically expressed in ampere-hours (Ah) or milliampere-hours (mAh) for smaller batteries.
- Nominal Capacity: Rated charge at standard conditions
- Actual Capacity: Real-world capacity under specific conditions
- Dependent on discharge rate, temperature
- Higher capacity = longer runtime for same load
Energy
Energy represents the total work a battery can perform, calculated as the product of voltage and capacity, measured in watt-hours (Wh) or kilowatt-hours (kWh) for larger battery storage systems.
- Energy = Voltage × Capacity
- Specific Energy: Energy per unit mass (Wh/kg)
- Energy Density: Energy per unit volume (Wh/L)
- Critical for battery storage system sizing
Additional Key Parameters
Power and Power Density
Power is the rate at which energy is delivered, measured in watts (W). It determines how quickly a battery can deliver energy to a load or accept energy during charging—critical for battery storage systems that need to respond quickly to demand changes.
- Power Density: Power per unit mass (W/kg) or volume (W/L)
- High power density = ability to deliver energy quickly
- Important for applications requiring rapid acceleration (EVs) or high bursts of power
- Different from energy density—batteries can excel at one but not the other
Internal Resistance
All batteries have some internal resistance that opposes current flow, measured in ohms (Ω). This resistance affects voltage under load and efficiency, particularly important in high-current battery storage applications.
- Voltage drop under load = Current × Internal Resistance
- Higher resistance = greater energy loss as heat
- Increases with age and temperature extremes
- Lower resistance is better for high-current applications
Cycle Life
Cycle life refers to the number of complete charge-discharge cycles a battery can undergo before its capacity drops to a specified percentage (typically 80%) of its original capacity. This is a critical factor in determining the economic viability of battery storage systems.
- Li-ion batteries: 500-2000+ cycles (varies by chemistry)
- Lead-acid batteries: 300-500 cycles
- Affected by depth of discharge, temperature, charge rate
- Shallower discharges generally extend cycle life
Charge and Discharge Rates
These rates indicate how quickly a battery can be charged or discharged, often expressed as a multiple of its capacity (C-rate). This is particularly important for battery storage systems that need to respond rapidly to grid demands.
- 1C rate = full charge/discharge in 1 hour
- 2C rate = full charge/discharge in 30 minutes
- Higher C-rates may reduce capacity and cycle life
- Different batteries have different optimal charge/discharge rates
Environmental and Operational Parameters
Temperature Range
Batteries operate within specific temperature ranges that significantly impact performance, safety, and lifespan. This is a critical consideration for battery storage systems deployed in extreme environments.
- Operating Temperature: Typically 0°C to 45°C for most batteries
- Storage Temperature: Usually wider range (-20°C to 60°C)
- Performance degrades at temperature extremes
- Charging at low temperatures can cause permanent damage
- High temperatures accelerate aging processes
Self-Discharge Rate
All batteries lose charge over time when not in use, known as self-discharge. This parameter is particularly important for battery storage systems that may remain idle for extended periods.
- Expressed as percentage of capacity lost per month
- Li-ion: 2-3% per month (low self-discharge)
- NiMH: 15-20% per month
- Lead-acid: 5-15% per month
- Increases significantly with temperature
- Primary batteries have very low self-discharge rates
Parameter Comparison Across Battery Chemistries
Different battery chemistries offer varying performance trade-offs, making certain technologies more suitable for specific applications—relevant to what is inside a battery charger. For example, lithium-ion batteries provide high energy density ideal for portable electronics and electric vehicles, while lead-acid batteries offer a lower-cost solution for applications where weight and size are less critical.
When designing battery storage systems, engineers must carefully balance these parameters based on application requirements. A grid-scale battery storage system might prioritize cycle life and cost over specific energy, while a battery for a portable device would emphasize energy density and compact size. Understanding these fundamental parameters is essential for selecting the right battery technology and optimizing system performance.Optical Transceiver.
Practical Implications for Battery Storage
For large-scale battery storage applications, certain parameters become particularly critical:
Summary of Key Parameters
Battery parameters define performance characteristics that determine suitability for specific applications. When evaluating battery storage solutions, consider the complete parameter set rather than individual specifications, as there are often trade-offs between different performance aspects.Electronic shelf labels.