Lithium Battery Overview
Lithium-ion batteries represent one of the most significant technological advancements in energy storage of the past few decades. Unlike traditional battery technologies, they offer high energy density, long cycle life, and relatively low self-discharge rates, making them ideal for a wide range of applications from portable electronics to electric vehicles and large-scale energy storage systems, including the increasingly popular lithium solar battery and solar battery bank installations that are revolutionizing renewable energy utilization.
The commercialization of lithium-ion batteries began in the early 1990s through the collaborative efforts of researchers at Sony and Asahi Kasei, building upon fundamental research conducted throughout the 1970s and 1980s. Since then, continuous innovation has led to significant improvements in energy density, power output, safety, and cost-effectiveness.
One of the key advantages of lithium-ion technology is its versatility. By varying the chemical composition of electrode materials, manufacturers can optimize batteries for specific applications—whether prioritizing energy density for electric vehicles, power delivery for industrial equipment, or cycle life for stationary storage systems like the solar battery bank that stores energy generated during daylight hours for use during evening and nighttime.
Today, lithium-ion batteries dominate the consumer electronics market and are rapidly becoming the standard for electric vehicles and renewable energy storage. Global production capacity has expanded exponentially to meet growing demand, with ongoing research focused on further improving performance characteristics while addressing challenges related to resource availability, recycling, and environmental impact throughout the battery lifecycle.

Lithium-ion Battery Evolution
Comparison of battery technologies showing energy density improvements over time, highlighting lithium-ion's advantages for modern applications including solar battery bank systems.
Key Advantages of Lithium-ion Technology
High Energy Density
Stores more energy per unit weight and volume compared to other rechargeable technologies, crucial for applications like electric vehicles and compact solar battery bank systems.
Long Cycle Life
Can undergo hundreds to thousands of charge-discharge cycles before significant capacity degradation, making them economical for long-term applications including solar battery bank installations.
Low Self-Discharge
Retains stored energy for longer periods when not in use compared to technologies like nickel-cadmium, an important feature for backup power and solar battery bank systems.
Working Principle of Lithium-ion Power Batteries
The answer to how does a battery work is reflected in the operation of a lithium-ion battery, which relies on the reversible movement of lithium ions between two electrodes during charge and discharge cycles. This fundamental mechanism enables the storage and release of electrical energy through chemical reactions, powering everything from smartphones to electric vehicles and large-scale storage solutions like the solar battery bank systems that integrate with renewable energy sources.
During discharge, lithium ions migrate from the negative electrode (anode) to the positive electrode (cathode) through an electrolyte medium, while electrons flow through an external circuit to power connected devices. This electron flow constitutes the electric current that performs work.
When charging, an external electrical power source reverses this process. The applied voltage drives lithium ions back from the cathode to the anode, where they are stored until the next discharge cycle. This reversible process is what distinguishes secondary (rechargeable) batteries from primary (single-use) batteries.
The separator, a porous membrane placed between the electrodes, allows lithium ions to pass through while preventing direct electrical contact between the anode and cathode that would cause a short circuit. The electrolyte, typically a lithium salt dissolved in an organic solvent, provides the medium through which lithium ions travel.
This basic principle operates consistently across all lithium-ion battery configurations, whether in small consumer electronics or large-scale applications such as the solar battery bank systems that store energy generated from photovoltaic panels for later use when sunlight is unavailable.
Lithium-ion Battery Operation
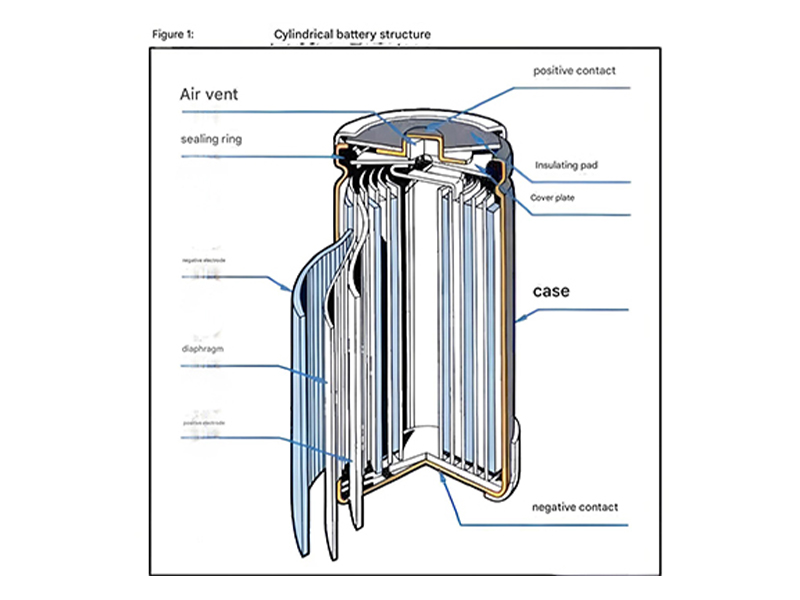
Charge and discharge cycle showing lithium ion migration between electrodes
Charge-Discharge Cycle Comparison
Voltage profile during typical charge and discharge cycles in a lithium-ion battery, similar to those used in solar battery bank applications.
Cathode Materials
The cathode material is a critical component of lithium-ion batteries, significantly influencing key performance characteristics including energy density, power output, cycle life, and safety. As the positive electrode where lithium ions are stored during charging, the cathode's chemical composition and structure determine many of the battery's properties, making material selection a key consideration for applications ranging from portable electronics to electric vehicles, solar panel battery, and solar battery bank systems.
Lithium cobalt oxide (LiCoO₂) was the first commercially successful cathode material, offering high energy density but with limitations in terms of safety and cycle life. It remains common in consumer electronics such as smartphones, laptops, and Optical Transceivers, but has been largely replaced in larger applications due to cost and safety concerns.
Lithium iron phosphate (LiFePO₄ or LFP) has gained popularity for its excellent safety profile, long cycle life, and lower cost due to the absence of cobalt. These characteristics make LFP particularly well-suited for stationary energy storage applications like solar battery bank installations, where safety and longevity are paramount.
Lithium nickel manganese cobalt oxides (NMC) represent a family of cathode materials with varying proportions of nickel, manganese, and cobalt. Higher nickel content increases energy density, making NMC batteries popular for electric vehicles. The balanced performance of NMC formulations also makes them suitable for certain solar battery bank applications requiring a combination of energy density and cycle life.
Other important cathode materials include lithium nickel cobalt aluminum oxide (NCA), favored for high-energy applications, and lithium manganese oxide (LMO), valued for its high rate capability. Ongoing research continues to develop new cathode materials and improve existing ones, focusing on increasing energy density, improving safety, extending cycle life, and reducing reliance on rare or expensive elements.
Cathode Material Comparison
Lithium Cobalt Oxide (LCO)
High energy density, lower safety, used in consumer electronics
Lithium Iron Phosphate (LFP)
Excellent safety and cycle life, ideal for solar battery bank systems
Nickel Manganese Cobalt (NMC)
Balanced performance, widely used in electric vehicles
Anode Materials
The anode, or negative electrode, plays a crucial role in lithium-ion battery performance by storing lithium ions during charging and releasing them during discharge. While cathode materials have historically received more attention, anode development has become increasingly important for improving overall battery performance, particularly in high-demand applications such as electric vehicles, solar storage battery, and solar battery bank systems where energy density and cycle life are critical factors.
Graphite is the most commonly used anode material in commercial lithium-ion batteries, offering several advantages including low cost, excellent cycling stability, and high coulombic efficiency (the ratio of charge extracted during discharge to the charge injected during charging). Graphite's layered structure allows lithium ions to intercalate between its carbon layers without significant volume change, contributing to its stability.
However, graphite has a theoretical capacity limitation of 372 mAh/g, which has prompted research into alternative materials with higher capacity. Silicon is one of the most promising alternatives, boasting a theoretical capacity approximately 10 times higher than graphite (4200 mAh/g). Silicon anodes can significantly increase battery energy density, but they face challenges related to large volume expansion during lithiation (up to 300%), which can cause electrode degradation and reduce cycle life.
Other advanced anode materials under investigation include various forms of carbon (such as hard carbon and carbon nanotubes), metal oxides, and metal alloys. These materials aim to address specific performance requirements, from increased capacity to improved rate capability or enhanced safety.
For stationary applications like solar battery bank systems, where weight considerations are less critical than cycle life and cost, graphite remains the dominant anode material due to its proven reliability and lower cost. As research progresses, hybrid anode designs combining graphite with higher-capacity materials are emerging to balance performance, cost, and durability across different application scenarios.

Anode Material Characteristics
Mature technology, low cost, excellent stability
High capacity, challenges with volume expansion
Higher capacity than graphite, good rate capability
Anode Selection Factors for Solar Battery Bank Systems
- Long cycle life (10,000+ cycles preferred)
- Low cost per kWh stored
- Stable performance across temperature ranges
- Minimal capacity fade over time
Failure Mechanisms of Lithium-ion Batteries
Understanding the failure mechanisms of lithium-ion batteries is crucial for improving their safety, reliability, and longevity across all applications, from consumer electronics and battery backup systems to large-scale energy storage systems like solar battery bank installations. Battery failure can occur through various interconnected processes, often accelerated by operating conditions such as temperature extremes, overcharging, deep discharge, or mechanical abuse.
Capacity fade, the gradual loss of a battery's ability to store energy, is one of the most common failure modes. This can result from several factors including the loss of active lithium ions through side reactions with the electrolyte, structural degradation of electrode materials, and the formation of a solid electrolyte interphase (SEI) layer that grows over time and consumes lithium ions.
Thermal runaway represents a more severe failure mode, involving an uncontrolled exothermic reaction that can lead to battery fires or explosions. This typically begins with a local temperature increase (thermal excursion) caused by internal short circuits, which may result from separator failure due to mechanical damage, dendrite growth (especially in lithium metal batteries), or electrode particle cracking.
For stationary applications like solar battery bank systems, calendar aging—capacity loss over time even when not in use—is a significant concern. This process is influenced by storage temperature, state of charge, and the chemical stability of battery materials. High temperatures accelerate calendar aging by increasing the rate of parasitic reactions between the electrodes and electrolyte.
Mechanical failure can occur due to physical damage, vibration, or thermal expansion/contraction cycles that stress battery components. In large battery packs like those used in solar battery bank installations, mechanical failure in one cell can propagate to adjacent cells, leading to cascading failures.
Mitigation strategies include advanced battery management systems (BMS) that control charging/discharging rates and prevent overcharging, thermal management systems to maintain optimal operating temperatures, and the development of more stable electrode materials and electrolytes. These measures are particularly important for ensuring the long-term reliability and safety of large-scale systems like solar battery bank installations, where battery replacement costs are significant.
Battery Failure Mechanisms

Thermal Runaway
Uncontrolled exothermic reactions leading to potential fire or explosion
Capacity Fade
Gradual loss of energy storage capability over charge cycles
Calendar Aging
Time-dependent degradation even when not in active use
Mechanical Failure
Physical damage to battery components affecting performance
Failure Prevention in Solar Battery Bank Systems
Thermal Management
Active and passive cooling systems maintain optimal operating temperatures (20-25°C), preventing accelerated aging and thermal runaway risks in solar battery bank installations.
Advanced BMS
Battery Management Systems monitor cell voltages, temperatures, and state of charge, preventing overcharging and deep discharge that accelerate failure.
Cell Balancing
Ensures uniform charge distribution across cells in a solar battery bank, preventing overcharging of individual cells that can lead to premature failure.
Predictive Maintenance
Monitoring systems analyze performance data to predict potential failures before they occur, allowing proactive maintenance of critical solar battery bank components.
Performance of Lithium-ion Power Batteries
The performance of lithium-ion power batteries is evaluated through several key metrics that determine their suitability for specific applications, from portable electronics to electric vehicles and stationary storage systems like solar battery bank installations—including solar power batteries. These performance characteristics are influenced by cell chemistry, design, manufacturing processes, and operating conditions.
Energy density, measured in watt-hours per kilogram (Wh/kg) or watt-hours per liter (Wh/L), represents the amount of energy a battery can store relative to its weight or volume. Higher energy density is particularly important for electric vehicles where weight and space are critical factors, while for solar battery bank systems, volume-specific energy density may be less important than overall capacity and cycle life.
Power density, measured in watts per kilogram (W/kg), indicates how quickly a battery can deliver its stored energy. High power density is essential for applications requiring rapid acceleration in electric vehicles or sudden power demand spikes in grid storage systems. Solar battery bank systems typically require moderate power density to handle daily charge-discharge cycles rather than extreme power demands.
Cycle life refers to the number of complete charge-discharge cycles a battery can undergo before its capacity drops to a specified percentage (usually 80%) of its initial capacity. For solar battery bank applications, long cycle life is critical to maximize return on investment, with modern systems often achieving 5,000 to 10,000 cycles or more under optimal conditions.
Charge and discharge rates, often expressed as multiples of the battery's capacity (C-rates), indicate how quickly a battery can be charged or discharged. A 1C rate means a battery can be fully charged or discharged in one hour. solar battery bank systems typically use lower charge rates (0.2C to 0.5C) to maximize cycle life, as slower charging reduces stress on battery materials.
Other important performance metrics include self-discharge rate (how quickly a battery loses charge when not in use), temperature performance range, efficiency (the ratio of energy output to energy input during a charge-discharge cycle), and cost per kilowatt-hour ($/kWh). For solar battery bank systems, round-trip efficiency (typically 80-95%) and lifecycle cost are particularly important economic considerations.
Battery Performance Metrics
Cycle Life Comparison
Optimal Performance Range for Solar Battery Bank Systems
Applications of Lithium-ion Power Batteries
Lithium-ion batteries have revolutionized numerous industries through their unique combination of high energy density, rechargeability, and relatively long cycle life. Their versatility has led to widespread adoption across applications ranging from small portable devices to large-scale energy storage systems, including the increasingly important solar battery bank installations that facilitate the integration of renewable energy into electrical grids.
In consumer electronics, lithium-ion batteries power smartphones, laptops, tablets, digital cameras, and wearable devices. Their high energy density allows for compact designs with extended usage times between charges, while their low self-discharge rate ensures devices retain charge when not in use—features that first established lithium-ion technology as a superior alternative to previous battery chemistries.
The transportation sector has seen transformative adoption of lithium-ion technology, particularly in electric vehicles (EVs). As automakers transition from internal combustion engines to electric propulsion, lithium-ion batteries provide the energy storage solution that balances range, weight, charging time, and lifespan requirements. Hybrid electric vehicles (HEVs), plug-in hybrid electric vehicles (PHEVs), and battery electric vehicles (BEVs) all rely on advanced lithium-ion battery systems optimized for automotive use.
One of the fastest-growing applications is stationary energy storage, including residential, commercial, and utility-scale systems. The solar battery bank has become a cornerstone of distributed renewable energy systems, storing excess electricity generated by solar panels during daylight hours for use during evening hours or cloudy periods. This application reduces reliance on grid electricity and maximizes the utilization of renewable energy.
Utility-scale lithium-ion battery installations provide grid stabilization services, frequency regulation, and load balancing, helping integrate intermittent renewable energy sources like wind and solar into the power grid. These large systems can respond to grid demands in milliseconds, making them valuable for maintaining grid stability.
Industrial applications include material handling equipment (such as forklifts), backup power systems for critical infrastructure, and off-grid power solutions for remote locations. In these settings, lithium-ion batteries offer advantages over traditional lead-acid batteries in terms of longer life, higher energy density, and lower maintenance requirements.
Emerging applications continue to expand the reach of lithium-ion technology, from maritime and aviation applications to grid-scale energy storage projects that enable higher penetration of renewable energy. The solar battery bank market, in particular, is projected to grow significantly as solar energy adoption increases and battery costs continue to decline, making renewable energy storage increasingly economically viable.Electronic shelf labels.

Residential Solar Battery Bank
Stores excess solar energy for household use, reducing electricity costs and providing backup power during outages.

Electric Vehicles
Power electric cars, buses, and trucks with high-energy-density batteries optimized for range and charging speed.

Utility-Scale Storage
Large installations providing grid stability, peak shaving, and integration of renewable energy sources.
Market Growth Projections for Key Applications
Projected growth of lithium-ion battery adoption across major application sectors, with significant expansion forecast for solar battery bank systems and electric vehicles.Related Hydraulic Spare Parts.